An Internal Combustion engine is comprised of an empty cylinder into which a piston is inserted. At the top of the piston, there are valves that inject a mixture of air and fuel into the cylinder which the piston compresses very tightly as it moves to fill the cylinder’s void space. When the volume of the air-fuel mixture in the cylinder is compressed down to a certain ratio, typically about 6:1 or 7:1, a spark is introduced thus explosively igniting the compressed air-fuel mixture. This drives the piston back down again. Horsepower is created by a crankshaft attached to the piston. So the fuel is matched to the compression ratio in the engine.
At low compression ratios, like 6:1, the fuel’s volatility increases making it more explosive. If you increased that ratio to 8:1, the fuel would spontaneously detonate just from the friction between its molecules being compressed by the piston. This is called pre-detonation or “knock.” It robs power from the engine and increases wear because the fuel is igniting before the piston reaches its full stroke.
Now, if you could increase compression to 10:1 or 12:1, the explosive detonation would greatly increase the power of the engine for each cycle of the piston. But 87 octane gas would explode on its own, at about 8:1. What was needed was a fuel with lower volatility that would remain stable when compressed to 1/1oth or 1/12th of its volume in the cylinder and not go off prematurely. That was what Doolittle wanted: Aviation fuel with an octane rating of 100 so that engine designers could make higher performance engines with higher compression ratios. The aircraft that carried these advanced engines would have stunning performance, especially at higher altitudes.
So Doolittle set about getting Shell Oil to make an engine fuel no one had yet manufactured. Yet, his farsightedness was not widely shared. Within Shell Oil, the project was being mocked as Doolittle’s “Million Dollar Blunder.”
For years Doolittle spent untold hours lobbying Congress and the Army to adopt a 100 octane fuel standard. The Army finally relented and adopted the 100 octane standard in 1938. Now Doolittle had a new problem: how to make 100 octane gas at a cost the government could afford. Initial experimental formulations of 100 octane fuel required a very expensive refining process that resulted in a prohibitively high fuel price of $25/gallon when automotive fuels were less than 20 cents a gallon. The thermal method of “cracking” high octane gas out of crude oil was wasteful and produced byproducts like olefins that gummed up engines. There was a solution to this problem, but it came from an unlikely place.

Eugene Houdry had served as a young officer in the French Tanks Corps in the First World War. He had been wounded in action, decorated for valor, awarded the Croix de Guerre, and made a Chevalier of the French Legion of Honour. After the war, Houdry took an interest in auto racing and visited the United States. He toured a Ford auto plant and attended the Indianapolis 500 race. What he saw in the U.S. was a country on the move. And the automobile was doing the moving.
America was producing its own oil and gas. On the other hand, France had to import virtually every drop of oil that fueled its military and civilian vehicles. Houdry saw this as a dangerous situation for France. Oil was becoming the lifeblood of modern economies and he thought he could help his country produce the gas and other fuels it needed.
Having earned a degree in mechanical engineering from the Ecole des Arts et Métiers in Chalons-sur-Marne Houdry set to work on converting brown coal (which France had in abundance) into fuel. He opened a small lab and by 1930 he had small samples of gasoline made from coal. This was considered pretty miraculous and Houdry became the pioneer of synthetic fuels. Soon Houdry was producing 60 tons of gasoline a day from coal using his revolutionary method. But the French government decided that importing oil was cheaper than making gas from coal and withdrew their funding of the Houdry process. France would pay dearly for relying on imported oil when WWII started.
But in America, Houdry’s work found a welcome reception from the Vacuum Oil Company (which later became Standard Oil). Houdry relocated to the U.S. and licensed his method in a joint venture to very cheaply produce high octane gas out of oil.
The venture started with a single Houdry Process plant in Marcus Hook, Pennsylvania. Soon there were 17 other plants in operation. Standard Oil had licensed the process to other oil companies like Sun Oil (Sunoco). It also licensed it to Shell Oil. There, Jimmy Doolittle immediately saw the promise of the Houdry Catalyst method of making the 100 octane gas he believed America would need if it ever fought a war again in Europe. And by 1937 this seemed increasingly likely.
After WWII began and France fell the Vichy government added insult to injury. It stripped Houdry of his citizenship for being a founding member of France Forever which sought to eject the German invader from the country. Houdry became a U.S. citizen and continued his work.
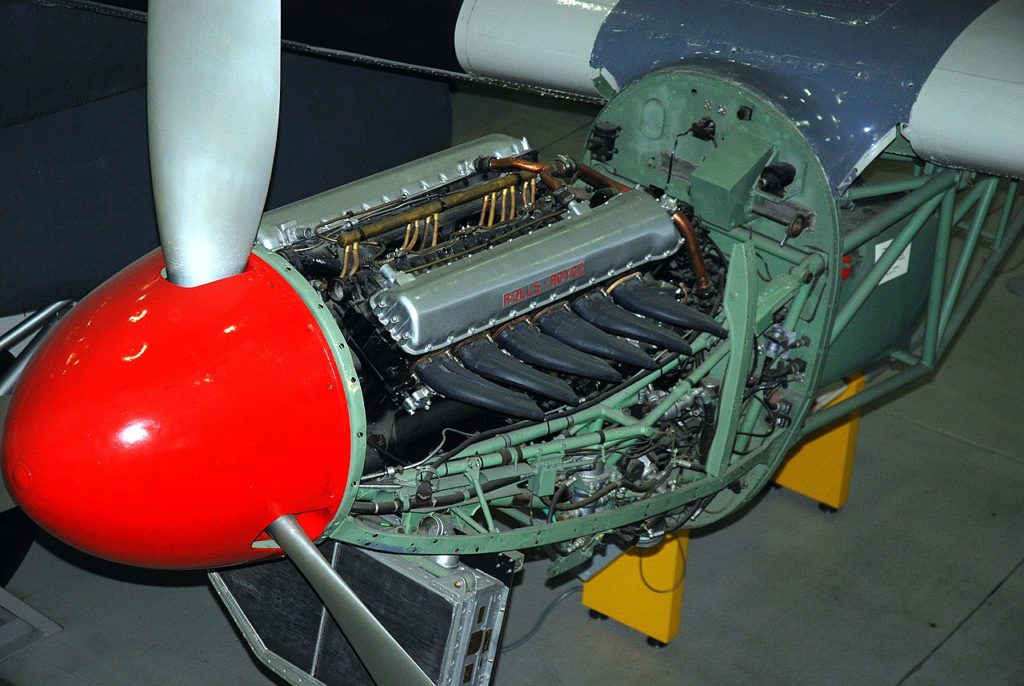
When the war in Europe began, the U.S. was manufacturing a modest 40,000 gallons a month of 100 octane gas. But by 1944, it was making 400,000 gallons of AvGas a month from 77 Houdry Process plants. The first consumer was Great Britain which did not make 100 octane gas but nevertheless made aircraft engines, like the Rolls Merlin, that needed it. The British were ordered all that the U.S. could produce.
And the Luftwaffe found that the underpowered Hurricanes and Spitfires it had slapped aside so easily in the skies over France early in the war had been reborn by the time the Battle of Brittain had started where they met or exceeded the performance of the Focke Wulfs and Messerschmitts they met in combat over London. In contrast, Germany’s planes mostly flew on 89 octane fuel and Japan never got higher than 87.

Fighter planes like the Spitfire, Mustang, Thunderbolt, Hurricane, SeaFury, Lightening, Hellcat, and Corsair all ran on 100 octane gas. This allowed the engines that powered them to attain stunning performance. The famous Rolls Merlin began the war producing just over 1,000 horsepower. By the war’s end, it was producing 1,600 horsepower. Spitfires could even approach the speed of sound in a shallow dive.
You may have read The Plane That Won the War or, The Best Aircraft Engine of WWII. Underlying it all was the contribution of 100 octane gas by a farsighted Doolittle, who understood the engineering science of airplane engines, and a maverick Frenchman who feared that disaster could befall his country if it relied solely on oil imports.
Their contributions made all the difference in making those planes and engines the legends they became and in ultimately achieving victory in WWII.
This article was originally published in December 2020.











COMMENTS